Draw a Diagram of Table Salt Nacl Dissolved in Water
CH 105 - Chemistry and Society
Intermolecular Forces (IMF) and Solutions
02/08/2008
Everyone has learned that there are 3 states of matter - solids, liquids, and gases. For the rest of the semester we will be discussing small molecules that are held together by covalent bonds, or ionic bonds. Given the property of solids, liquids (take shape container, tin can be poured, etc) and gases (fill their container), we surmised that the molecules in a solid and liquid must attract each other, with forces that are much weaker than the forces which attract atoms to each other within a molecule - such as covalent bonds. These INTERMOLECULAR attractive forces must be stronger in solids, weaker in liquids, and mostly nonexistent in gases.
Using water equally an example, we reviewed how solids could be convert to liquids and and then to gases. Indeed, as we saw in the guide on atoms and atomic structure, each land tin exist interconverted to the others.
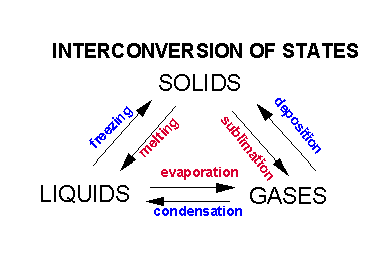
It requires energy in the form of heat to change water from a solid to liquid and then to a gas. This free energy is required to interruption upwards the intermolecular forces which hold the water molecules together. What is the nature of these intermolecular forces?
To answer this question, let'southward compare the properties of several pairs of molecules. These properties tin to a meaning degree be determined from the Lewis structures of the molecules.
COii and H2O, each with iii atoms -
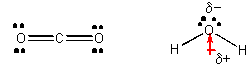
From the Lewis structures we can determine that the geometry of carbon dioxide atoms is linear but the geometry of the water atoms is angular. This leads to the prediction that CO2 is nonpolar but H2O is. It has a permanent dipole. Since all interactions in chemical science are essentially electrostatic in origin, we would hypothesize that IMFs would also arise to some kind of electrostatic interactions. Polar water molecules should concenter each other more than strongly than the nonpolar carbon dioxide molecules concenter each other. This would lead u.s.a. to further hypothesize that water has a loftier melting signal (MP) and boiling indicate (BP) than CO2. This is truthful. Dry out ice, which is CO2(s), actually sublimes (turns directly from a solid to a gas) at a temperature much below 0oC., while water melts at 0oC. It takes much less free energy to changed states in a substance in which the IMF's are weak than in a substance that tin attract other similar molecules with stronger IMF's. If water were linear instead of bent, it would take a very low MP and BP and not exist in the liquid state at room temperature, making life on globe incommunicable.
Let's take a closer look as to how water molecules attract each other. H is the smallest of all atoms. When covalently bonded to O is has a clear δ+ charge. Because information technology is so pocket-size, information technology can become very close to an oxygen atom on another h2o molecule. It really can go very shut to a solitary pair of electrons on the other O atom. Attractions between + and - and δ+ and δ- depends on how close they become. The closer, the stronger the attractions. Since H is so small and can get so close to a lone pair on an oxygen on some other water molecule, the interactions betwixt the δ+ on H and δ- on an O are strong (simply much weaker than a covalent bond). This type if intermolecular force is called a hydrogen bond (H-bail).
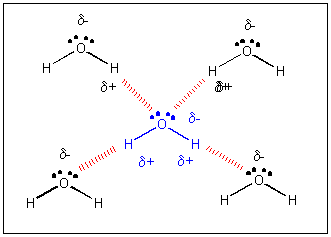
H-bonds can from between an H on a(north) F, O, or N on one molecule, and a partially negative F, O, or N on another molecule. For instance, H-bonds tin can course betwixt NH3 and H2O, between HF and HiiO, but not between F2 and H2O since the F atoms in Fii are not slightly negative or positive since the bond between them is nonpolar covalent. A dissimilar way to consider an H bond is that described past Atkins:
"A hydrogen bond is a link formed by a (slightly positive) hydrogen atom lying between ii strongly electronegative atoms." (This would include an H bond between the H on water and a Cl- ion, for example.)
H2O and (CH3)2CO (acetone), each a liquid at room temperature -
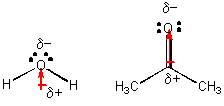
A quick demonstration shows that acetone evaporates much more than quickly than h2o, suggesting that the International monetary fund amid acetone molecules are weaker than among h2o molecules. Both of these molecules are polar, as illustrated above. and then what is the departure? Hopefully you tin can encounter that water molecules tin can attract each other through H-bonds, but acetone tin can't since it has no H's that are bonded to F, O, Northward, or Cl - i.e. there are no slightly positive H atoms. Acetone molecules attract each other since they are both have permanent dipoles. This blazon of Imf, which is weaker than H bonds, is called dipole-dipole interactions. You might besides be able to imagine an ion - dipole interaction
- dipole - dipole interaction (top flash diagram)
- ion - dipole (H bail)
- Ethanol/Water Solubility: Blitheness
North2 and NaCl, each with 2 atoms (or ions) -
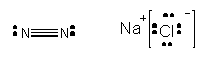
Apparently, North2 exists equally a gas at room temperature while NaCl is a solid. Conspicuously, the Imf's between molecules of NaCl in a crystals of NaCl are much stronger than for N2. Liquid nitrogen be, just boils at a temperature of -196oC. The difference tin exist explained by viewing a model of the crystal construction of NaCl.
- NaCl Jmol model
- Jmol models of other inorganic compounds
. Each Na+ was surrounded by 6 Cl- and vice versa. The are very strong Imf'southward between "molecules" of NaCl in the solid. If you isolate one molecule of NaCl in the crystal structure, information technology is attracted to other NaCl "molecules" in they solid by ion-ion International monetary fund. This type of International monetary fund conspicuously is stronger than a H-bond since the attractions are between fully charged ions, not partially charged atoms. In contrast, North2 is non polar and has no permanent dipole. Hence these molecules are attracted to each other weakly. But y'all know they notwithstanding attract each other since liquid nitrogen exists. What is the ground for this interaction?
- ion - ion interaction
If all bonny interactions ascend from accuse interactions, then we might speculate that somehow a temporary development of partial accuse might develop in nitrogen molecules. You lot could image this happening in the following ways. Remember, in contrast to our Lewis structures of molecules which show electrons equally static bonds or lonely pairs, the electrons are actually moving all effectually the nuclei. They most probably are symmetrically distributed around the molecule. However, at whatever requite fourth dimension, they would accept a probability of being non-symmetrically distributed. For example, at ane example, more than of the electrons might be at one end of molecule, giving information technology a slight negative accuse and the opposite end a slight positive charge. That is, a instantaneous dipole is formed. If at that moment, another nitrogen atoms approaches, the slight positive end of the first nitrogen molecule would attract the electron deject of the 2d, creating a temporary induced dipole in that molecule, which would permit both molecules to be attracted to each other. This weak IMF is called an induced dipole-induced dipole IMF. It also goes by ii other names, Van der Waals forces (VDW) or London Force.
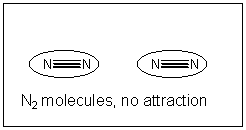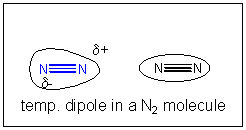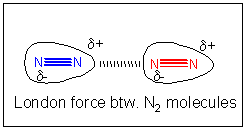
- VDW FORCES: ANIMATION/Explanation
London forces are the only interaction that exist between all species, including ions, polar molecules, and nonpolar molecules. London interactions between polar molecules is unremarkably stronger than their dipole-dipole interactions. This tin be seen in the trend in boiling points in HCl, HBr, and HI. Although HCl is more polar than the others, it has a lower BP. HI has the highest BP in this series, because of its large number of electrons, and greater London forces.
The case with acetone above is only partially true. In addition to dipole-dipole interactions, at that place are more electrons in acetone than water, which would permit greater London forces between acetone molecules than among h2o molecules. Acetone molecules are attracted past both dipole-dipole interactions and London forces. The force of the H-bonds among h2o molecules still predominates in determining the higher boiling point of water compared to acetone. Other types of mixed interactions can also occur.
CHiv and C8H18, each containing but C and H -
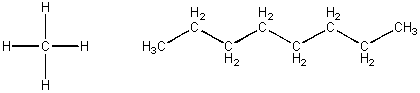
These molecules are both nonpolar and each would attract a like molecule through London forces. The first molecule, methane, is a gas at room temperature. The 2d, octane, is a liquid at RT and a component of gasoline. Octane molecules must attract each other with potent London forces than exercise methane molecules. This suggests that the bigger the molecules, the neat chance for induced dipoles forming when similar molecules approach. Since all Imf arise from the allure of + (full, or slight) and - (full or slight), the larger molecule must have more slight + and - interactions another large molecule than occur between two modest molecules. With larger molecules, there is greater surface area for these weak attractive forces to piece of work. The table beneath describes the different types of IMFs and how much free energy (kcal is a unit of energy and mol is a a fixed number of such interactions) is required to intermission the IMFs.
| Imf | Energy (kcal/mol) | Interacting Species |
| ion-ion | 60 | ions merely |
| H-bonds | 4-5 | FON on 1 molecule and |
| ion-dipole | 3.5 | ions and polar molecules |
| dipole-dipole | 0.5-1 | polar molecules |
| London | 0.v | all types of molecules |
See the links below for visual applications of IMFs
![]()
Quiz: Intermolecular Forces 1
Solutions
About all the chemistry that we will study this year deals with reactions of molecules in aqueous solution. This too applies to reactions in the body, which consists of greater than threescore% by weight of h2o. Before we written report solutions, nosotros demand to review our definition of a solution. In our first unit on matter, we defined solutions as homogeneous mixtures - In homogenous mixtures, the particles are and so small that they never carve up on standing or in simple centrifugation, and practise not interfere with calorie-free passing through the mixture. Hence the mixture appears clear. The solution can not be separated into its component parts past filtration. It can exist separated past other physical techniques like chromatography (every bit in lab 1), distillation, etc. If we sample a given solution at different locations, it will accept the same composition at every location.
The material that dissolves in a liquid is called the solute. The liquid that dissolves the solute is called the solvent. Of course we can have solution of solids (similar salt), liquids (like ethanol) and gases (like carbon dioxide) - all solutes - dissolved in the liquid solvent. Likewise the air is a solution of gas solutes in a gas solvent. Some substance tin dissolve in water, others can't. Substances that tin can't deliquesce in h2o often dissolve in other solvents. Some solid substances dissolve in a solvent similar water to different extents. When no more solute can exist added to a solvent, we say the solution is saturated with the solute. Whatever additional solid added will remain as a solid in the solution.
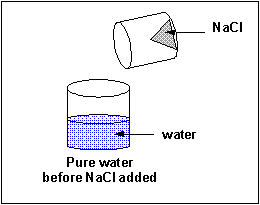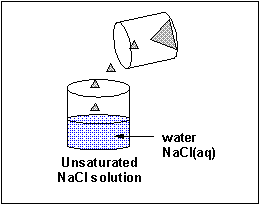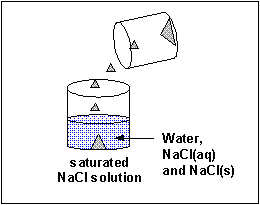
A liquid solute may not deliquesce in water. In this case, it will course a discrete layer either higher up or below the h2o layer (depending on its density). Such a liquid is immiscible in the solvent. Some liquid solvents deliquesce in whatsoever proportion in h2o. Such a liquid solute is completely miscible.
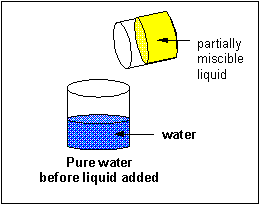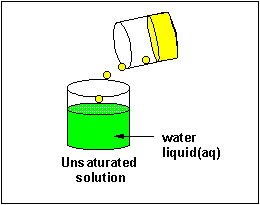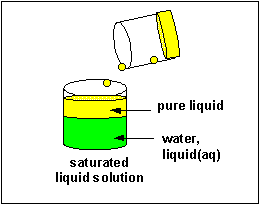
Solubilty of molecules in water and hexane
A noesis of IMF's can assist united states of america empathize the solubility of different substances (solutes) in different liquids (solvents). In form I did a series of demonstrations which showed if different solutes were soluble in ii dissimilar solvents, h2o and hexane, whose structures are shown below.
A quick inspection of these solvents show them to exist quite different. hexane, a clear, colorless liquid at room temperature, is completely nonpolar and interacts with other hexane molecules through London forces. In contrast, water, a clear, colorless liquid at room temperature, is polar and interacts with other water molecules through H-bonds. You might expect they might dissolve different types of solutes. Explore the solubility differences of the unlike solutes below in hexane and h2o. Develop a law that will allow you to predict the solubility of a substance in water or hexane, and so develop an hypothesis that helps explain the law.
1. NaCl - sodium chloride:
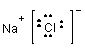
NaCl is a common salt held together by ionic bonds. In a sense it is as polar a substance equally y'all can get. It dissolves in water (as you know from feel) but non in hexane.
- Animation: NaCl dissolves in h2o from Iowa State

2. Itwo - Iodine:
![]()
Iodine, a covalent solid, in dissimilarity to water, is completely nonpolar. Information technology dissolves in hexane to produce a imperial-colored solution. Information technology didn't dissolve in water.
TENTATIVE LAW: From the higher up two examples, nosotros can surmise that polar molecules dissolve in polar liquids, and non-polar molecules in nonpolar liquids.
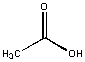
Acetic acrid was soluble in h2o and insoluble in hexane. An inspection of the molecule shows that is is predominately polar with a modest nonpolar CH3 group. Our tentative law seems to work so far!
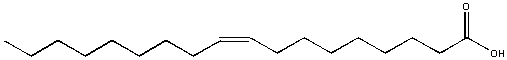
Oleic acrid, found in olive oil, is soluble in hexane only non soluble in water. It formed a carve up layers in water. In this case, about of the molecule is nonpolar (the long C-H tail) with just a modest part at the right finish being polar. The nonpolar sections wins out and determines its solubility in water.
MODIFIED TENTATIVE Law: From the above examples, nosotros tin can surmise that molecules dissolve in polar liquids if the solute is predominately polar, or in nonpolar solvents if the solute is predominately nonpolar.
![]()
Methanol, a clear, colorless liquid, dissolved in water but merely slightly dissolved in hexane. Once more this molecule is predominately polar so our modified law is supported. Please note that methanol dissolves but does not form ions in solution.

![]()
Octanol, a mostly nonpolar molecule, dissolves in hexane but formed a carve up layer in water. Once more our modified law is upheld.
We can use our tentative police force to predict whether the following substances are soluble in water or hexane.
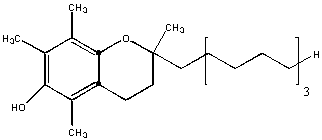
This molecule is almost entirely nonpolar. Hence information technology will exist insoluble in water and soluble in hexane. The is an instance of a fat-soluble vitamin. One early definition of fatty is biological molecules that are soluble in organic solvents like hexane.
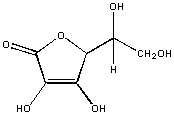
In vitamin C, every C is fastened to an oxygen atoms. This molecule is polar and volition dissolve in water, but not hexane.
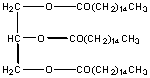
This molecule is also a fat and is the predominant type of fat stored in fat tissue in your body. At that place are 9 polar carbons, only 42 non polar C's. It is insoluble in water and soluble in hexane. Triglycerides are the major type of substance in vegetable oils.
We get in at the following conclusions:
- 'like dissolves like"
- substances that are mostly polar (like glucose) dissolve in polar solvents like water but not in a nonpolar solvent like hexane, presumably since the dipole forces (specifically H-bonds) belongings polar solute molecules together can be replaced with polar solute-solvent interactions (H-bonds).
- substances that are by and large nonpolar dissolve ion nonpolar solvents similar hexane but not in polar solvents like water, presumably since the London forces holding nonpolar solute molecules together tin can exist replaced with nonpolar solute-solvent interactions.
- Molecules that are both about equal amount of polar and nonpolar parts may or maynot dissolve readily in different solvents.
Call up, Only BECAUSE A SOLUTE DISSOLVES IN H2o DOESN'T IMPLY THAT IT DISSOCIATES INTO IONS Like SOLUBLE SALTS AND STRONG ACIDS!
Image: How methanol, CH3OH dissolves in water.
Yous should now be able to predict the solubility of different substances in water and other solvents. Take for instance O2. Would you lot expect this to exist very soluble in water? It'southward not since information technology is nonpolar and can't form ion-ion, H-bonds, or dipole-dipole interactions with h2o. We tin't get by past "breathing water" since not enough O2 tin be dissolved in water. That'southward why we have a protein in our blood called hemoglobin which specifically binds and carries O2, finer increasing the "dissolved" concentration of oxygen by 100 fold. Given this info, how can you explain the photograph below:
-
OF MICE AND FISH?
-
Another view
-
A novel treatment
![]()
Quiz: Intermolecular Forces two and Solutions
Micelles and Bilayers
We have seen that the solubility properties of molecules strongly depend on how much of the molecule is polar and how much is nonpolar. Consider stearic acid, shown below, which has 18 carbons. The end containing the two oxygens (shown in red) is polar, but the remainder of the molecule is completely nonpolar (shown in blueish). We tin depict a "drawing" model of this model as a circle - representing the polar finish or "caput group" with a single connecting line - representing the long, nonpolar "tail".

Pretend you are that molecule with your head representing the polar head grouping and the rest of your body the nonpolar tail. Now dive into water. How would yous orient yourself in h2o? In that location are several possible ways. A small number of these molecules might be soluble in h2o (remember even insoluble salts dissociate to a modest degree to course a few ions). Mostly the nonpolar tail wants to go out of the water, while the polar head like to stay in the water. In that location are two ways this can be done. Some of the molecules migrate to the surface of the water, with the nonpolar tails sticking out into air, away from h2o, to form a monolayer on the top of the water. Others volition self-aggregate, through Imf's to form a spherical construction in which the nonpolar tails are sequested from water and the polar caput are facing the water. This construction is called a micelle. Detergents consist of molecules with very similar structure to stearic acid pictured to a higher place. They form micelles in water. Grease from clothes or foods, unremarkably not soluble in water, can "dive" into the middle of the micelle and be carried off by this structure. Find that at that place is not water within the micelle as you tin see by selecting Micelles below.
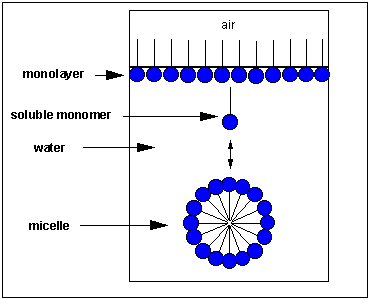
-
Claret substitutes
-
Solubility of Oxygen
-
Structure of perfluorocarbons
![]() micelle
micelle ![]() micelle Jmol
micelle Jmol
At present consider another molecule somewhat similar to stearic acid, called a phoshpolipid. A typical phospholipid structure is shown beneath. Notice it has a polar end (shown in red), but in dissimilarity to stearic acid above, it has 2 long nonpolar tails shown in blue. We can draw a "cartoon" model of this as a circumvolve - representing the polar end or "head group" with two connecting lines - representing the two long nonpolar "tails".

How would you orient this molecule in water? At that place are several possible ways. A small number of these molecules might be soluble in water equally higher up. Mostly, withal, the nonpolar tails wants to get out of the water, while the polar head like to stay in the water. Once again, some of the molecules drift to the surface of the h2o, with the nonpolar tails sticking out into air, away from h2o, to form a monolayer on the tiptop of the water. Others will self- amass, through IMF's to form a bilayer or membrane. Because at that place are two tails per head group, the tails can't pack together equally tightly. Imagine the bilayer or membrane curving effectually and eventually meeting. A structure like this would await similar a small-scale biological prison cell. In dissimilarity in a micelle, the interior of this little cell, or liposome, is filled with water which can interact through IMF'south to the caput groups of the inner leaflet of this membrane. The caput groups of the outer leaflet of the membrane interact through IMF's with the bulk water.
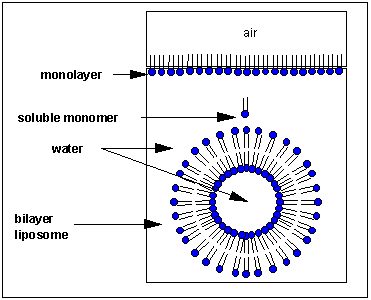
![]() bilayer
bilayer ![]() | Jmol
| Jmol
Phospholipids are the basic component of really biological membranes. Liposomes are useful since they are simple models of actual biological membranes. In addition, they are starting to be used therapeutically. Toxic drugs, like those used in chemotherapy, tin be incorporated into the aqueous book inside a liposome where they tin be targeted to specific tumor cells.
Liposomes and micelles seem to be complicated structures. Notwithstanding their formation and existence can be predicted from the simple solubility backdrop of these molecules and the concepts of IMF'southward.
Source: https://employees.csbsju.edu/hjakubowski/classes/Chem%20and%20Society/IMF_Solutions/olIMF_solutions.htm
0 Response to "Draw a Diagram of Table Salt Nacl Dissolved in Water"
Post a Comment